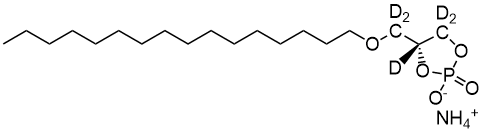
857328 | 18:1 Cyclic LPA
1-oleoyl-sn-glycero-2,3-cyclic-phosphate (ammonium salt)

18:1 Cyclic LPA
1-oleoyl-sn-glycero-2,3-cyclic-phosphate (ammonium salt)
CAS Registry Number is a Registered Trademark of the American Chemical Society
Fujiwara Y. Cyclic phosphatidic acid - a unique bioactive phospholipid. Biochim Biophys Acta. 2008 Sep;1781(9):519-24. doi: 10.1016/j.bbalip.2008.05.002. Epub 2008 May 23. PMID: 18554524; PMCID: PMC2572151.
PubMed ID: 18554524Murakami-Murofushi K, Kaji K, Kano K, Fukuda M, Shioda M, Murofushi H. Inhibition of cell proliferation by a unique lysophosphatidic acid, PHYLPA, isolated from Physarum polycephalum: signaling events of antiproliferative action by PHYLPA. Cell Struct Funct. 1993 Oct;18(5):363-70. doi: 10.1247/csf.18.363. PMID: 8168160.
PubMed ID: 8168160Mukai M, Imamura F, Ayaki M, Shinkai K, Iwasaki T, Murakami-Murofushi K, Murofushi H, Kobayashi S, Yamamoto T, Nakamura H, Akedo H. Inhibition of tumor invasion and metastasis by a novel lysophosphatidic acid (cyclic LPA). Int J Cancer. 1999 Jun 11;81(6):918-22. doi: 10.1002/(sici)1097-0215(19990611)81:6<918::aid-ijc13>3.0.co;2-e. PMID: 10362139.
PubMed ID: 10362139Fischer DJ, Liliom K, Guo Z, Nusser N, Virág T, Murakami-Murofushi K, Kobayashi S, Erickson JR, Sun G, Miller DD, Tigyi G. Naturally occurring analogs of lysophosphatidic acid elicit different cellular responses through selective activation of multiple receptor subtypes. Mol Pharmacol. 1998 Dec;54(6):979-88. doi: 10.1124/mol.54.6.979. PMID: 9855625.
PubMed ID: 9855625Fujiwara Y, Sebök A, Meakin S, Kobayashi T, Murakami-Murofushi K, Tigyi G. Cyclic phosphatidic acid elicits neurotrophin-like actions in embryonic hippocampal neurons. J Neurochem. 2003 Dec;87(5):1272-83. doi: 10.1046/j.1471-4159.2003.02106.x. PMID: 14622107.
PubMed ID: 14622107- Certificate of Analysis (Lot No. 857328P-1MG-D-013 and 5815PGD013)
- Certificate of Analysis (Lot No. 857328P-1MG-E-013 and 5815PGE013)
- Certificate of Analysis (Lot No. 857328P-1MG-A-014 and 5815PGA014)
- Certificate of Analysis (Lot No. 857328P-1MG-B-014 and 5815PGB014)
- Certificate of Analysis (Lot No. 857328P-1MG-C-014 and 5815PGC014)