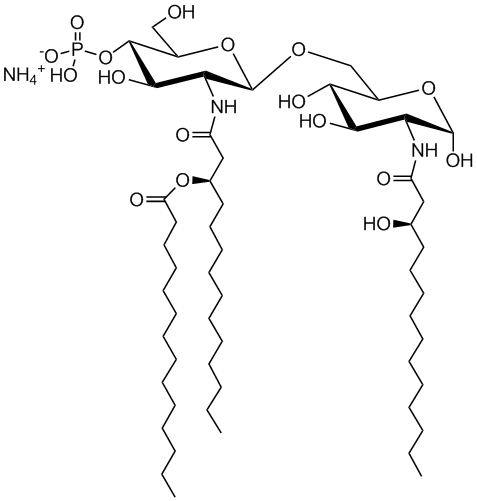
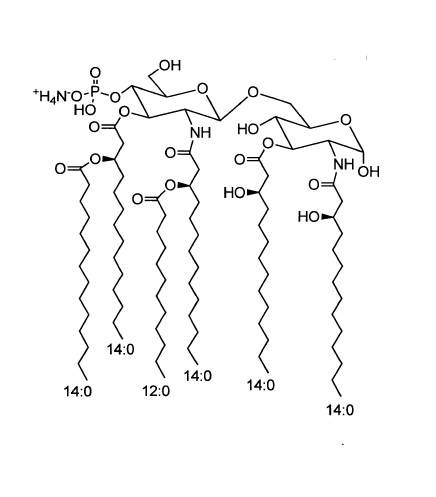
699855 | 3D-(6-acyl) PHAD®
Monophosphoryl Hexa-acyl Lipid A, 3-Deacyl (Synthetic)

3D-(6-acyl) PHAD®
Monophosphoryl Hexa-acyl Lipid A, 3-Deacyl (Synthetic)
Vaccination is well-accepted as an effective method to prevent infections by mounting pathogen-specific immune responses prior to the infection. Usually, immunization with vaccine antigens alone is not able to induce robust or long-lasting immune responses — resulting in failure of protective immunity against infections. Thus, adjuvants are required to enhance cellular or humoral immune responses upon immunization. Because vaccine adjuvants using Lipid A have proven to be safe and effective in inducing Th-1 type immune responses to heterologous proteins in animal and human vaccines, Avanti developed Phosphorylated HexaAcyl Disaccharide (PHAD®), the first fully synthetic monophosphoryl Lipid A available for use as an adjuvant in human vaccines.
The MPLA structural analog, 3D-(6-acyl)-PHAD® , is the synthetic MPLA most closely related to the reported structure of MPL® Adjuvant used in GSK’s Adjuvant Systems AS01, AS02, and AS04. As with other synthetic MPLA analogs manufactured by Avanti, it is structurally homogeneous and highly purified, and mimics the TLR4 agonist activity of bacterial MPLA.
Stimulatory effect of PHAD®, 3D-PHAD®, and 3D-(6-acyl)-PHAD® on macrophages. Macrophage cell line J774 cells were cultured with Avanti PHAD®, 3D-PHAD®, or 3D-(6-acyl)-PHAD® for 24hrs. IL-12 levels in supernatants were measured by sandwich ELISA.
Adjuvant Activity

PHAD®, 3D-PHAD®, and 3D-(6A)-PHAD® have been tested extensively in animals using a variety of antigens. In all cases, these adjuvants exhibit a similar activity and safety profile to bacterially-derived MPL. The data above demonstrate the equivalency of the three synthetic adjuvants to the bacterially-derived MPL when presented in a liposomal carrier system (DMPC/DMPG/Cholesterol).
CAS Registry Number is a Registered Trademark of the American Chemical Society
Shao S, Huang WC, Lin C, Hicar MD, LaBranche CC, Montefiori DC, Lovell JF. An Engineered Biomimetic MPER Peptide Vaccine Induces Weakly HIV Neutralizing Antibodies in Mice. Ann Biomed Eng. 2019 Dec 12;10.1007/s10439-019-02398-8. doi: 10.1007/s10439-019-02398-8. [Epub ahead of print]. PMID: 31832930.
PubMed ID: 31832930Hernandez A, Luan L, Stothers CL, Patil NK, Fults JB, Fensterheim BA, Guo Y, Wang J, Sherwood ER, Bohannon JK. Phosphorylated Hexa-Acyl Disaccharides Augment Host Resistance Against Common Nosocomial Pathogens. Crit Care Med. 2019 Nov;47(11):e930-e938. doi: 10.1097/CCM.0000000000003967.
PubMed ID: 31567352Taleghani N, Bozorg A, Azimi A, Zamani H. Immunogenicity of HPV and HBV vaccines: adjuvanticity of synthetic analogs of monophosphoryl lipid A combined with aluminum hydroxide. APMIS. 2019 Mar;127(3):150-157. doi: 10.1111/apm.12927.
PubMed ID: 30746792- Certificate of Analysis (Lot No. 699855P-1MG-A-010 and 5091PGA010)
- Certificate of Analysis (Lot No. 699855P-5MG-A-010 and 5091PHA010)
- Certificate of Analysis (Lot No. 699855P-1MG-B-010 and 5091PGB010)
- Certificate of Analysis (Lot No. 699855P-5MG-B-010 and 5091PHB010)
- Certificate of Analysis (Lot No. 699855P-CONF-A-010 and 5091PWA010)
- Certificate of Analysis (Lot No. 699855P-1MG-C-010 and 5091PGC010)
- Certificate of Analysis (Lot No. 699855P-5MG-C-010 and 5091PHC010)
- Certificate of Analysis (Lot No. 699855P-5MG-D-010 and 5091PHD010)
- Certificate of Analysis (Lot No. 699855P-5MG-E-010 and 5091PHE010)
- Certificate of Analysis (Lot No. 699855P-5MG-F-010 and 5091PHF010)
- Certificate of Analysis (Lot No. 699855P-1MG-D-010 and 5091PGD010)
- Certificate of Analysis (Lot No. 699855P-5MG-G-010 and 5091PHG010)
- Certificate of Analysis (Lot No. 699855P-1MG-E-010 and 5091PGE010)
- Certificate of Analysis (Lot No. 699855P-5MG-A-011 and 5091PHA011)
- Certificate of Analysis (Lot No. 699855P-1MG-A-011 and 5091PGA011)
- Certificate of Analysis (Lot No. 699855P-5MG-C-011 and 5091PHC011)
- Certificate of Analysis (Lot No. 699855P-1MG-B-011 and 5091PGB011)
- Certificate of Analysis (Lot No. 699855P-5MG-E-011 and 5091PHE011)
- Certificate of Analysis (Lot No. 699855P-1MG-C-011 and 5091PGC011)
- Certificate of Analysis (Lot No. 699855P-1MG-E-011 and 5091PGE011)
- Certificate of Analysis (Lot No. 699855P-1MG-D-011 and 5091PGD011)
- Certificate of Analysis (Lot No. 699855P-5MG-G-011 and 5091PHG011)